



|
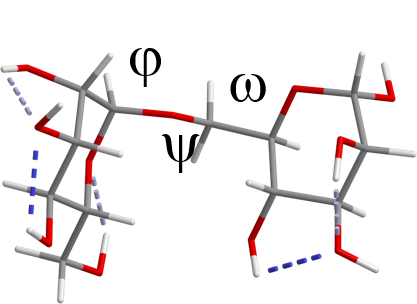
Solution Conformation of Carbohydrates by NMR: Compounds with hydroxyl clusters, such as carbohydrates, have potentially biologically interesting properties. They have been implicated in a multitude of biochemical processes that are central to life on earth. The structural diversity among carbohydrates and - as a consequence - their potential to "code" for biochemical information has no parallel. It is, therefore, striking that nature only uses a comparatively small number (~ a few thousand) of structures to make biochemistry on this planet possible. The accurate description of carbohydrate conformation in aqueous solution is critical before function can be understood. The solution conformation is complicated by many different factors. We use NMR spectroscopy and theoretical methods to develop an acceptable quantitiative description. Hydrogen Bonding in Carbohydrates: Exchangeable protons contribute to hydrogen bonding during biochemical recognition events. However, exchangeable protons are difficult to detect by NMR spectroscopy. We use H/D-exchange techniques and the resulting chemical isotope shift effects at variable temperatures to detect evidence for transient hydrogen bonding. Oligosaccharide Tags: Glycans play important roles in inflammation, fertilization, immunocompatibility, and cell growth. The inherent difficulty in detecting oligosaccharides released from glycoproteins due to the absence of chromophores has been addressed in the past by introducing UV-active or fluorescent labels into the sugar prior to chromatographic separation. We develop and evaluate new labeling reagents for oligosaccharides. The glycan derivatives are purified and studied by MALDI-TOF mass spectrometry. Collaborative projects with Dr. George Pantouris and Skylar Carlson |
|
|
INTERESTED TO JOIN?
|
College of the Pacific | Pharmaceutical Sciences and Chemistry
Updated: Apr. 2025